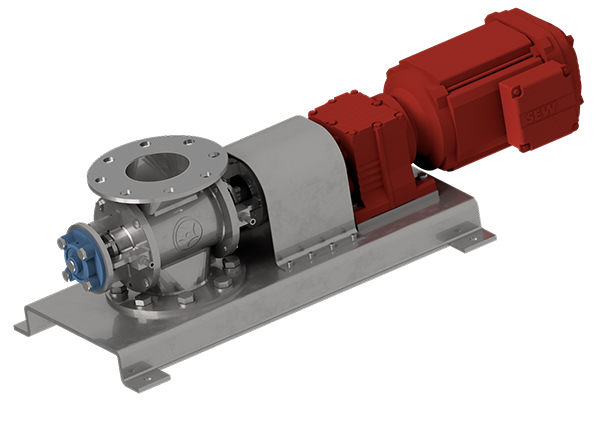
FDA and EC 1935/2004 compliant rotary valves are used in the food and pharmaceutical industries as well as in biotechnology and genetic engineering for metering, conveying and discharging bulk materials and as a protective system or device in potentially explosive atmospheres. Design according to Machinery Directive 2006/42/EC or according to ATEX Directive 2014/34/EU.
FDA-compliant rotary valves can also be supplied with polished surfaces with surface roughnesses below 1.0 μm, rounded chamber plates, GMP and CIP-compliant design.
Type
- Drive via directly flanged geared motor or with free shaft end
- With motor lantern, torque support or base plate
- With rotor extraction device as ZRS EASYCLEAN
- Sealing via radial shaft seals or packing glands
- Housing as cast or welded construction
- With round or angular inlet and outlet
- Cellular impellers with up to 14 chambers
- Surface primed, painted, blasted or chemically nickel-plated
- Structurally safe
- Pressure shock resistant up to 25 bar according to RL2014/34/EU
- Pressure shock resistant and ignition proof up to 10 bar according to RL2014/34/EU